Description
Transfection reagent features:
- Efficient delivery of DNA, shRNA, siRNA or oligonucleotides in vivo
- 200 ®l reagent for 20 transfections in mouse
- Efficient delivery to the lung, liver, kidney, and spleen, liver, pancreas and certain type of tumor via systemic administration
- Delivery via Multiple modes of administration in many species
- Avalanche®-in vivo/nucleic acid conjugated complexes are stable in serum for 16h
- No detectable inflammatory responses
- Efficient siRNA and plasmid DNA delivery via direct subcutaneous tumor injection (multiple tumor types)
- Reproducible results
- Applicable for plasmid DNA/siRNA co-injection
- Developed and manufactured by EZ Biosystems LLC
Data
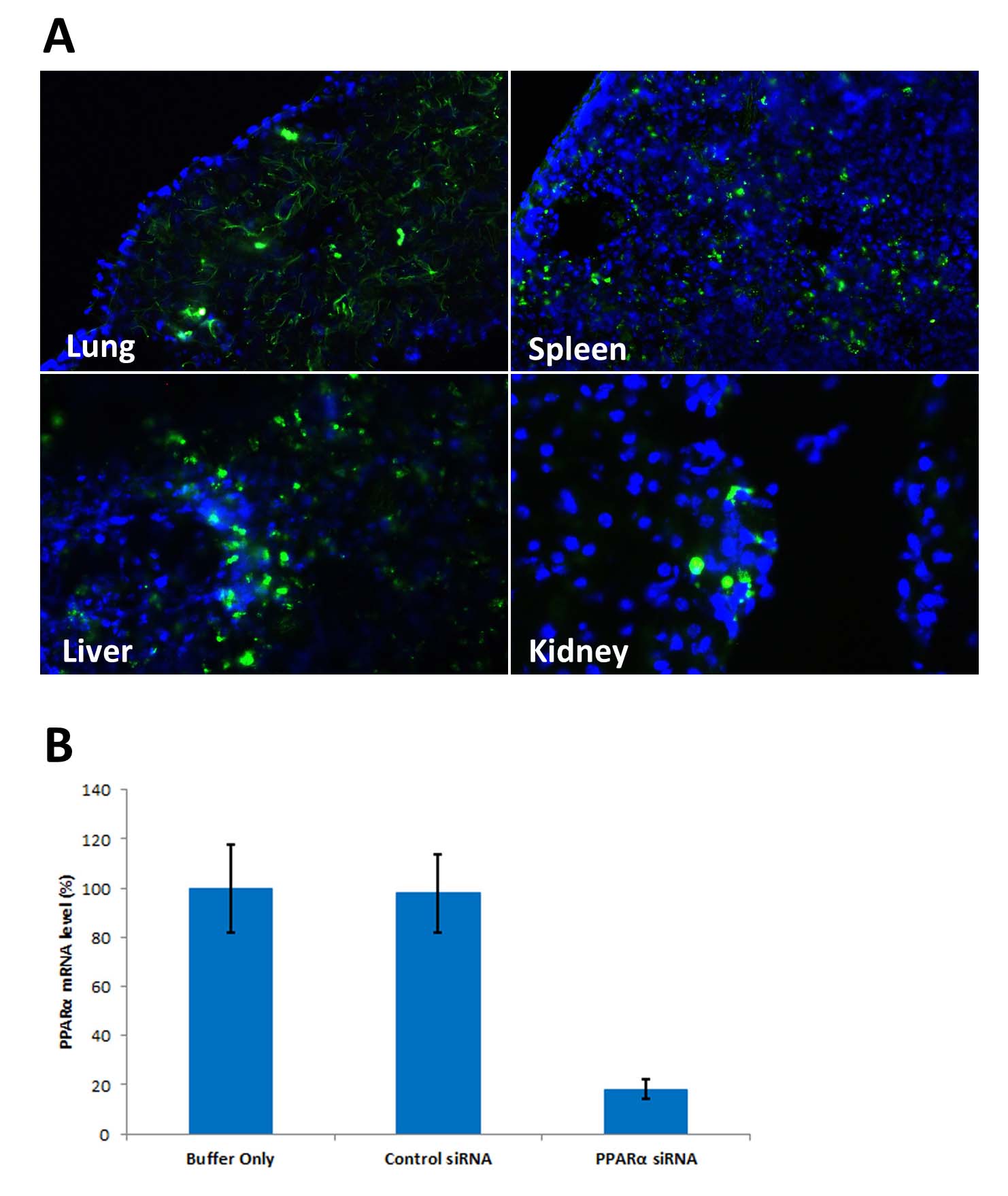 Figure 1. Avalanche®-in vivo Transfection Reagent performs high transfection efficiency in vivo in mice. (A). Avalanche®-in vivo Transfection Reagent successfuly delivered EGFP expression vectors into diffeent organs via systemic administration (tail vein). 48 hours post transfection, different organs were harvested. Frozen sections revealed EGFP positive cells. DAPI was used to stain nucleus. (B)In vivo RNAi test: Systemic administration (i.v.) of Avalanche®-in vivo Transfection Reagent conjugated with siRNA targeting PPARa mRNA or non-silencing control siRNA (30 ®g/mouse) following the recommended protocol. Liver was collected and RNA isolated 24 hours after first injection. Samples were analyzed by qRT-PCR for PPARa mRNA expression levels. Ribosomal RNA levels were used to normalize the PPARa mRNA data. Data are means +/- SD (n=6).
Figure 1. Avalanche®-in vivo Transfection Reagent performs high transfection efficiency in vivo in mice. (A). Avalanche®-in vivo Transfection Reagent successfuly delivered EGFP expression vectors into diffeent organs via systemic administration (tail vein). 48 hours post transfection, different organs were harvested. Frozen sections revealed EGFP positive cells. DAPI was used to stain nucleus. (B)In vivo RNAi test: Systemic administration (i.v.) of Avalanche®-in vivo Transfection Reagent conjugated with siRNA targeting PPARa mRNA or non-silencing control siRNA (30 ®g/mouse) following the recommended protocol. Liver was collected and RNA isolated 24 hours after first injection. Samples were analyzed by qRT-PCR for PPARa mRNA expression levels. Ribosomal RNA levels were used to normalize the PPARa mRNA data. Data are means +/- SD (n=6).
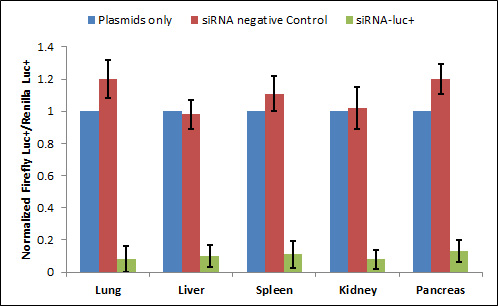
Figure 2. Co-transfection application: RNA interference in organs of mice after transfection of siRNA using Avalanche®-in vivo Transfection Reagent. Mice were co-injected with the pGL3 control vector and pRL-SV40 plasmids (Promega) alone or together with siRNAs. The two plasmids contain genes of P. pyralis luciferase+ (Pp-luc+) and R. reniformis luciferase (Rr-luc) respectively. In all cases, the ratio of Pp-luc+ to Rr-luc activities was calculated to compensate for differences in transfection efficiencies between mice. The ratios were then normalized to those observed in mice receiving no siRNA. Blue bars, red bars, and green bars indicate normalized Pp-luc+/Rr-luc activity ratios in mice receiving plasmids only, in controls co-injected with siRNA-EGFP, and in those co-injected with siRNA-luc+ respectively (References method: Lewis et al., Nature Genetics 32: 107-8, 2002, but used Avalanche®-in vivo Transfection Reagent for transfection).
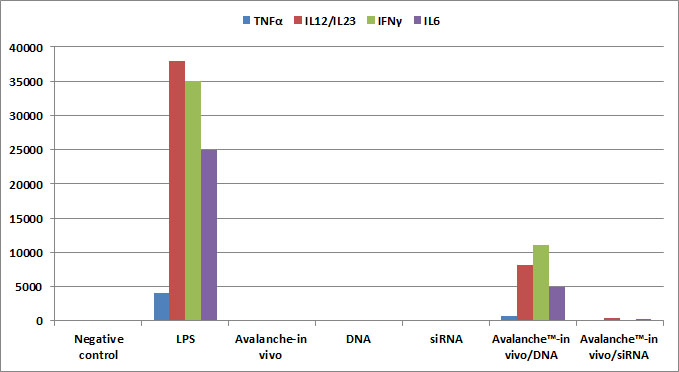 Figure 3. Avalanche®-in vivo does not cause any significant pro-inflammatory responses: Serum concentration of TNF-a, IL12/IL23, IFNr and IL6 following intravenous nucleic acid delivery using Avalanche®-in vivo Transfection Reagent 6 h after delivery. The negative control consists of an IV injection of 5% glucose; the positive control an IP injection of 50 ®g LPS. 40 ®g DNA and 40 ®g siRNA were delivered with or without Avalanche®-in vivo Transfection Reagent.
Figure 3. Avalanche®-in vivo does not cause any significant pro-inflammatory responses: Serum concentration of TNF-a, IL12/IL23, IFNr and IL6 following intravenous nucleic acid delivery using Avalanche®-in vivo Transfection Reagent 6 h after delivery. The negative control consists of an IV injection of 5% glucose; the positive control an IP injection of 50 ®g LPS. 40 ®g DNA and 40 ®g siRNA were delivered with or without Avalanche®-in vivo Transfection Reagent.
Additional Information
| Weight | 0.5 lbs |
|---|---|
| Product Sizes |
0.2 ml, 0.6 ml |
| Subcategories |
in vivo |
Documents
Protocols
MSDS
Citations or Feedback
- Baghel, K. S., Tewari, B. N., Shrivastava, R., Malik, S. A., Lone, M. U., Jain, N. K., . . . Bhadauria, S. (2016). Macrophages promote matrix protrusive and invasive function of breast cancer cells via MIP-1beta dependent upregulation of MYO3A gene in breast cancer cells. Oncoimmunology, 5(7), e1196299. doi:10.1080/2162402x.2016.1196299
- Hartigan-O”Connor, D. J. (2023). CONJUGATE POLYPEPTIDES AND VACCINES FOR INDUCING IMMUNE RESPONSES. USPTO(18/055,808). Retrieved from https://patents.google.com/patent/US20230087396A1/en
- Herbert, A. G. (2019). METHODS AND COMPOSITIONS TO INDUCE OR SUPPRESS IMMUNE RESPONSES THROUGH THE USE OF MEMBRANE BOUND COMPLEMENT SPLIT PRODUCTS. (PCT / US2019 / 057993). Retrieved from https://patents.google.com/patent/WO2020092140A2/en
- Shimoda, H., Doi, S., Nakashima, A., Sasaki, K., Doi, T., & Masaki, T. (2019). inhibition of the H3K4 methyltransferase MLL1/WDR5 complex attenuates renal senescence in ischemia reperfusion mice by reduction of p16(INK4a). Kidney Int, 96(5), 1162-1175. doi:10.1016/j.kint.2019.06.021