Description
Transfection reagent features:
- One single reagent is able to transfect large plasmid, mRNA, siRNA, and/or other type of nucleic acids, which is best for co-transfection of different type and/or size of nucleic acids.
- Exceptional transfection efficiency in the broadest range of cell types including cell lines of stem cell origin, suspension cells, and primary cells with less than half the amount required by competitors® products.
- Extremely gentle to cells: The chemical features of our proprietary formula and the least amount needed for transfection ensure its lowest toxicity.
- Economical: High efficiency means less amount of nucleic acid & reagent is needed. 1.5 ml of Avalanche®-Omni Reagent is sufficient for more than 3,500 transfections in 24-well plates.
- Superior performance for co-transfection of siRNA and plasmid DNA
- Proven efficacy in the presence of serum: eliminates the need to change media following transfection
- Animal free
- Reliable performance for high-throughput applications
- The best choice for establishing stable cell lines
- Simplest protocol: simply mix with nucleic acid and add to cell culture.
Data
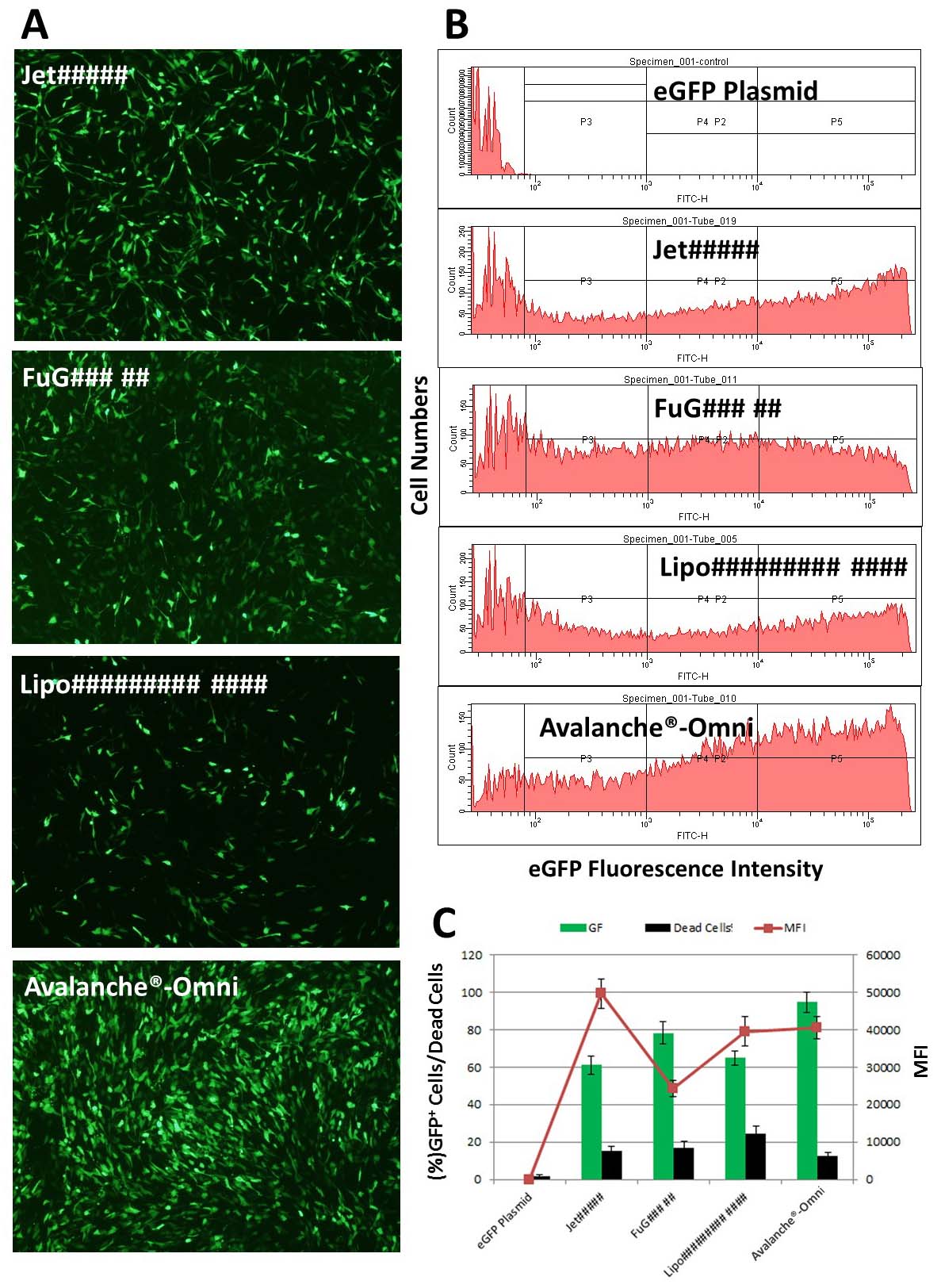 Figure 1. Avalanche®-Omni Transfection Reagent revealed highest efficiency and optimal balance of potency & low cytotoxicity. NIH 3T3 cells were transfected with enhanced green fluorescent protein (eGFP) expressing vector by using our Avalanche®-Omni Transfection Reagent and several most popular commercial transfection reagents. The transfection result was evaluated by FACS using three criteria: the percent of GFP positive cells (transfection efficiency), the percent of dead cells (toxicity, 7AAD positive cells), and mean fluorescence intensity (MFI, expression level of GFP in cells).(A) Pictures of GFP expression in cells transfected with Avalanche®-Omni and other commercial transfection reagents. (B) FACS data showed the diffeences on transfection efficiency between Avalanche®-Omni and other commercial transfection reagents. (C) Quantitative data of (B) FACS analysis: Avalanche®-Omni showed the highest transfection efficiency and the optimal balance of potent & low cytotoxicity. GFP expression level in cells are high as well in Avalanche®-Omni Transfection Reagent group.
Figure 1. Avalanche®-Omni Transfection Reagent revealed highest efficiency and optimal balance of potency & low cytotoxicity. NIH 3T3 cells were transfected with enhanced green fluorescent protein (eGFP) expressing vector by using our Avalanche®-Omni Transfection Reagent and several most popular commercial transfection reagents. The transfection result was evaluated by FACS using three criteria: the percent of GFP positive cells (transfection efficiency), the percent of dead cells (toxicity, 7AAD positive cells), and mean fluorescence intensity (MFI, expression level of GFP in cells).(A) Pictures of GFP expression in cells transfected with Avalanche®-Omni and other commercial transfection reagents. (B) FACS data showed the diffeences on transfection efficiency between Avalanche®-Omni and other commercial transfection reagents. (C) Quantitative data of (B) FACS analysis: Avalanche®-Omni showed the highest transfection efficiency and the optimal balance of potent & low cytotoxicity. GFP expression level in cells are high as well in Avalanche®-Omni Transfection Reagent group.
Avalanche®-Omni Transfection Reagent is able to achieve more than 90% knockdown of endogenous gene expression in a variety of cell lines and primary cells.
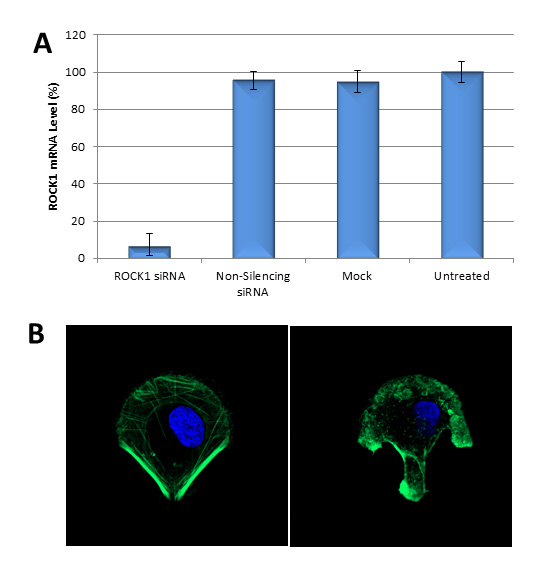 Figure 2. Avalanche®-Omni Transfection Reagent performed more than 90% knockdown of endogenous gene expression. (A). ROCK1 mRNA levels were quantified using qPCR in Hela cells transfected with Target-specific siRNA duplexes (10nM) for human ROCK1 gene or non-silencing siRNA by using Avalanche®-Omni Transfection Reagent. Data were normalized against the 18S rRNA signal. Control Samples were either mock-transected or untreated. Values are normalized to untreated sample. Data are means®SD (n=3). (B) Actin stress fibers were stained with FITC-labelled phalloidin on the cells in (A) cultured on fibronectin-coated micropattern. Confocal Microscope revealed that the one transfected with ROCK1 siRNA (right) showed disrupted stress fiber pattern as compared to the one transfected with non-silencing siRNA (left).
Figure 2. Avalanche®-Omni Transfection Reagent performed more than 90% knockdown of endogenous gene expression. (A). ROCK1 mRNA levels were quantified using qPCR in Hela cells transfected with Target-specific siRNA duplexes (10nM) for human ROCK1 gene or non-silencing siRNA by using Avalanche®-Omni Transfection Reagent. Data were normalized against the 18S rRNA signal. Control Samples were either mock-transected or untreated. Values are normalized to untreated sample. Data are means®SD (n=3). (B) Actin stress fibers were stained with FITC-labelled phalloidin on the cells in (A) cultured on fibronectin-coated micropattern. Confocal Microscope revealed that the one transfected with ROCK1 siRNA (right) showed disrupted stress fiber pattern as compared to the one transfected with non-silencing siRNA (left).
Additional Information
| Weight | 0.5 lbs |
|---|---|
| Product Sizes | 0.75 ml, 1.5 ml, 5 x 1.5 ml |
| Subcategories | Broad Spectrum |
Documents
Protocols
MSDS
Citations or Feedback
- Agbaegbu Iweka, C., Hussein, R. K., Yu, P., Katagiri, Y., & Geller, H. M. (2021). The lipid phosphatase-like protein PLPPR1 associates with RhoGDI1 to modulate RhoA activation in response to axon growth inhibitory molecules. J Neurochem, 157(3), 494-507. doi:10.1111/jnc.15271
- Ajuh, E. T., Wu, Z., Kraus, E., Weissbach, F. H., Bethge, T., Gosert, R., . . . Hirsch, H. H. (2018). Novel Human Polyomavirus Noncoding Control Regions Differ in Bidirectional Gene Expression according to Host Cell, Large T-Antigen Expression, and Clinically Occurring Rearrangements. J Virol, 92(7). doi:10.1128/jvi.02231-17
- Byrne, D. J., Garcia-Pardo, M. E., Cole, N. B., Batnasan, B., Heneghan, S., Sohail, A., . . . O’Sullivan, N. C. (2022). Liver X receptor-agonist treatment rescues degeneration in a Drosophila model of hereditary spastic paraplegia. Acta Neuropathol Commun, 10(1), 40. doi:10.1186/s40478-022-01343-6
- Chang, J., Lee, S., & Blackstone, C. (2014). Spastic paraplegia proteins spastizin and spatacsin mediate autophagic lysosome reformation. J Clin Invest, 124(12), 5249-5262. doi:10.1172/jci77598
- Chen, J., Harding, S. M., Natesan, R., Tian, L., Benci, J. L., Li, W., . . . Greenberg, R. A. (2020). Cell Cycle Checkpoints Cooperate to Suppress DNA- and RNA-Associated Molecular Pattern Recognition and Anti-Tumor Immune Responses. Cell Rep, 32(9), 108080. doi:10.1016/j.celrep.2020.108080
- Chesnel, F., Jullion, E., Delalande, O., Couturier, A., Alusse, A., Le Goff, X., . . . Arlot-Bonnemains, Y. (2022). Mutation of the proline P81 into a serine modifies the tumour suppressor function of the von Hippel-Lindau gene in the ccRCC. Br J Cancer, 127(11), 1954-1962. doi:10.1038/s41416-022-01985-2
- Chu, Y., Cohen, B. E., & Chuang, H. H. (2020). A single TRPV1 amino acid controls species sensitivity to capsaicin. Sci Rep, 10(1), 8038. doi:10.1038/s41598-020-64584-2
- Dong, Y., Furuta, T., Sabit, H., Kitabayashi, T., Jiapaer, S., Kobayashi, M., . . . Nakada, M. (2017). Identification of antipsychotic drug fluspirilene as a potential anti-glioma stem cell drug. Oncotarget, 8(67), 111728-111741. doi:10.18632/oncotarget.22904
- Egner, J. M., Nolden, K. A., Harwig, M. C., Bonate, R. P., De Anda, J., Tessmer, M. H., . . . Hill, R. B. (2022). Structural studies of human fission protein FIS1 reveal a dynamic region important for GTPase DRP1 recruitment and mitochondrial fission. J Biol Chem, 298(12), 102620. doi:10.1016/j.jbc.2022.102620
- Goetz, C. J., Sprague, D. J., & Smith, B. C. (2020). Development of activity-based probes for the protein deacylase Sirt1. Bioorg Chem, 104, 104232. doi:10.1016/j.bioorg.2020.104232
- Greenberg, E., Hochberg-Laufer, H., Blanga, S., Kinor, N., & Shav-Tal, Y. (2019). Cytoplasmic DNA can be detected by RNA fluorescence in situ hybridization. Nucleic Acids Res, 47(18), e109. doi:10.1093/nar/gkz645
- Harwig, M. C., Viana, M. P., Egner, J. M., Harwig, J. J., Widlansky, M. E., Rafelski, S. M., & Hill, R. B. (2018). Methods for imaging mammalian mitochondrial morphology: A prospective on MitoGraph. Anal Biochem, 552, 81-99. doi:10.1016/j.ab.2018.02.022
- Higashi, S. L., Yagyu, K., Nagase, H., Pearson, C. S., Geller, H. M., & Katagiri, Y. (2020). Old but not obsolete: an enhanced high-speed immunoblot. J Biochem, 168(3), 313. doi:10.1093/jb/mvaa078
- Jadhao, S. J., & Anderson, L. J. (2016). Detection of RSV Antibodies in Human Plasma by Enzyme Immunoassays. Methods Mol Biol, 1442, 41-52. doi:10.1007/978-1-4939-3687-8_4
- Kasahara, Y., Osuka, S., Takasaki, N., Bayasula, Koya, Y., Nakanishi, N., . . . Kajiyama, H. (2021). Primate-specific POTE-actin gene could play a role in human folliculogenesis by controlling the proliferation of granulosa cells. Cell Death Discov, 7(1), 186. doi:10.1038/s41420-021-00566-1
- Katagiri, Y., Morgan, A. A., Yu, P., Bangayan, N. J., Junka, R., & Geller, H. M. (2018). Identification of novel binding sites for heparin in receptor protein- tyrosine phosphatase (RPTPsigma): Implications for proteoglycan signaling. J Biol Chem, 293(29), 11639-11647. doi:10.1074/jbc.RA118.003081
- Kelly, C. M., Byrnes, L. J., Neela, N., Sondermann, H., & O’Donnell, J. P. (2021). The hypervariable region of atlastin-1 is a site for intrinsic and extrinsic regulation. J Cell Biol, 220(11). doi:10.1083/jcb.202104128
- Kim, J. W., Jung, S. Y., Kim, Y., Heo, H., Hong, C. H., Seo, S. W., . . . Chang, J. (2021). Identification of Cathepsin D as a Plasma Biomarker for Alzheimer’s Disease. Cells, 10(1). doi:10.3390/cells10010138
- Lazarou, M., Sliter, D. A., Kane, L. A., Sarraf, S. A., Wang, C., Burman, J. L., . . . Youle, R. J. (2015). The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature, 524(7565), 309-314. doi:10.1038/nature14893
- Lee, S., Chang, J., & Blackstone, C. (2016). FAM21 directs SNX27-retromer cargoes to the plasma membrane by preventing transport to the Golgi apparatus. Nat Commun, 7, 10939. doi:10.1038/ncomms10939
- Liu, T. Y., Chu, Y., Mei, H. R., Chang, D., & Chuang, H. H. (2019). Two Vanilloid Ligand Bindings Per Channel Are Required to Transduce Capsaicin-Activating Stimuli. Front Mol Neurosci, 12, 302. doi:10.3389/fnmol.2019.00302
- Maeda, T. K., Sugiura, D., Okazaki, I. M., Maruhashi, T., & Okazaki, T. (2019). Atypical motifs in the cytoplasmic region of the inhibitory immune co- receptor LAG-3 inhibit T cell activation. J Biol Chem, 294(15), 6017-6026. doi:10.1074/jbc.RA119.007455
- Maruhashi, T., Okazaki, I. M., Sugiura, D., Takahashi, S., Maeda, T. K., Shimizu, K., & Okazaki, T. (2018). LAG-3 inhibits the activation of CD4(+) T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat Immunol, 19(12), 1415-1426. doi:10.1038/s41590-018-0217-9
- Mencio, C. P., Tilve, S. M., Suzuki, M., Higashi, K., Katagiri, Y., & Geller, H. M. (2022). A novel cytoskeletal action of xylosides. PLoS One, 17(6), e0269972. doi:10.1371/journal.pone.0269972
- Nakamura, T., & Tsuchiya, M. (2021). Effects of NAD+ Synthesis Levels on Sirtuin 1 Deacetylase Activity in Mammalian Cells. Shimane Journal of Medical Science, 38(2), 59. Retrieved from https://www.jstage.jst.go.jp/article/sjms/38/2/38_59/_pdf
- Nakane, K., Nagasawa, H., Fujimura, C., Koyanagi, E., Tomoshige, S., Ishikawa, M., & Sato, S. (2022). Switching of Photocatalytic Tyrosine/Histidine Labeling and Application to Photocatalytic Proximity Labeling. Int J Mol Sci, 23(19). doi:10.3390/ijms231911622
- Nakatani, T., Tsujimoto, K., Park, J., Jo, T., Kimura, T., Hayama, Y., . . . Kumanogoh, A. (2021). The lysosomal Ragulator complex plays an essential role in leukocyte trafficking by activating myosin II. Nat Commun, 12(1), 3333. doi:10.1038/s41467-021-23654-3
- Nezich, C. L., Wang, C., Fogel, A. I., & Youle, R. J. (2015). MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and Atg5. J Cell Biol, 210(3), 435-450. doi:10.1083/jcb.201501002
- Nord, J. A., Wynia-Smith, S. L., Gehant, A. L., Jones Lipinski, R. A., Naatz, A., Rioja, I., . . . Smith, B. C. (2022). N-terminal BET bromodomain inhibitors disrupt a BRD4-p65 interaction and reduce inducible nitric oxide synthase transcription in pancreatic beta- cells. Front Endocrinol (Lausanne), 13, 923925. doi:10.3389/fendo.2022.923925
- Osborn, J. L., & Greer, S. F. (2015). Metastatic melanoma cells evade immune detection by silencing STAT1. Int J Mol Sci, 16(2), 4343-4361. doi:10.3390/ijms16024343
- Renvoise, B., Malone, B., Falgairolle, M., Munasinghe, J., Stadler, J., Sibilla, C., . . . Blackstone, C. (2016). Reep1 null mice reveal a converging role for hereditary spastic paraplegia proteins in lipid droplet regulation. Hum Mol Genet, 25(23), 5111-5125. doi:10.1093/hmg/ddw315
- Sugiura, D., Maruhashi, T., Okazaki, I. M., Shimizu, K., Maeda, T. K., Takemoto, T., & Okazaki, T. (2019). Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science, 364(6440), 558-566. doi:10.1126/science.aav7062
- Sugiura, D., Okazaki, I. M., Maeda, T. K., Maruhashi, T., Shimizu, K., Arakaki, R., . . . Okazaki, T. (2022). PD-1 agonism by anti-CD80 inhibits T cell activation and alleviates autoimmunity. Nat Immunol, 23(3), 399-410. doi:10.1038/s41590-021-01125-7
- Takanezawa, Y., Nakamura, R., Matsuda, H., Yagi, T., Egawa, Z., Sone, Y., . . . Kiyono, M. (2019). Intracellular Demethylation of Methylmercury to Inorganic Mercury by Organomercurial Lyase (MerB) Strengthens Cytotoxicity. Toxicol Sci, 170(2), 438-451. doi:10.1093/toxsci/kfz094
- Tilve, S., Iweka, C. A., Bao, J., Hawken, N., Mencio, C. P., & Geller, H. M. (2020). Phospholipid phosphatase related 1 (PLPPR1) increases cell adhesion through modulation of Rac1 activity. Exp Cell Res, 389(2), 111911. doi:10.1016/j.yexcr.2020.111911
- Toyotome, T., Takahashi, H., & Kamei, K. (2016). MEIS3 is repressed in A549 lung epithelial cells by deoxynivalenol and the repression contributes to the deleterious effect. J Toxicol Sci, 41(1), 25-31. doi:10.2131/jts.41.25
- Tsujimoto, K., Jo, T., Nagira, D., Konaka, H., Park, J. H., Yoshimura, S. I., . . . Kumanogoh, A. (2023). The lysosomal Ragulator complex activates NLRP3 inflammasome in vivo via HDAC6. EMBO J, 42(1), e111389. doi:10.15252/embj.2022111389
- Zhang, J., Furuta, T., Sabit, H., Tamai, S., Jiapaer, S., Dong, Y., . . . Nakada, M. (2020). Gelsolin inhibits malignant phenotype of glioblastoma and is regulated by miR-654-5p and miR-450b-5p. Cancer Sci, 111(7), 2413-2422. doi:10.1111/cas.14429


